Knowledge of physical properties is essential for the design of innovative chemical products and processes. It would be very difficult to select a pump without knowing the liquid's density and viscosity, or design a distillation column without knowing component vapor pressures and activity coefficients, or a coating without understanding the mixture's viscosity.
Very often, especially in those early conceptual stages of a new process or product design, we may not know the physical properties of the chemicals and mixtures we are working with. This is when physical property estimation can be very useful. Often from only the knowledge of a chemical's molecular structure or a mixture's component, we can use estimation techniques to obtain a good estimates, good enough for our design calculations.
If you are searching for physical property data and estimates, visit the web pages listed to the right. The "Data and Estimates" page contains data on more than 2,000 chemicals. This web page also provides you with estimates as functions of temperature and pressure. The "Introduction" page enables you to draw the molecular structure of a chemical and estimate its physical properties using group-contribution and equation-based techniques.
If you are interested in learning more about any particular estimation techniques, e.g., learning about how accurate a technique might be, click on a technique name below. The technique's webpage will provide you with background information, error estimates, and the ability to use an online version of the technique.
Finally, if you have any suggestions for additional data or techniques, or any general suggestions, comments, or questions, please contact us. We are also happy to talk about physical properties.
Thank you, Kevin G. Joback
President
Molecular Knowledge Systems, Inc.
clicking here.
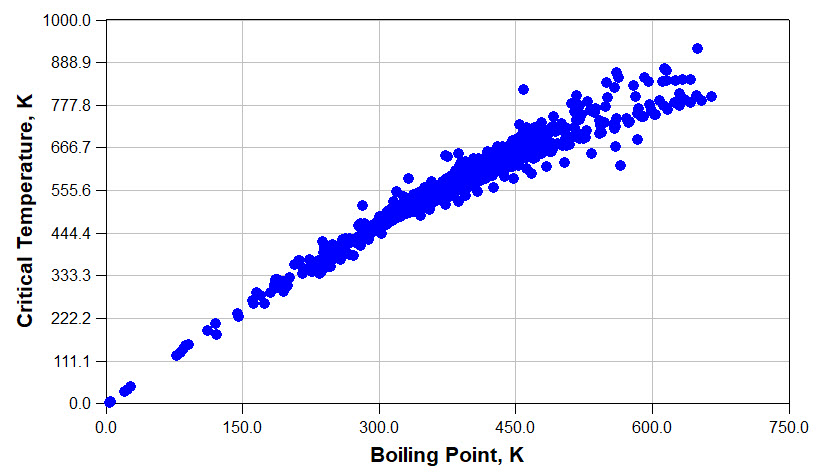
clicking here.
We are continually adding new estimation techniques to both Cranium and Synapse. Below is a listing of the current techniques included in the MKS Core Knowledge Base. Click on the technique's name to see more details including implementation notes and evaluation statistics. (As always, please contact us to request the addition of new techniques.)